EluNIR – PERL™
Ridaforolimus Eluting Coronary Stent System
EluNIR-PERL is Medinol’s most recent advancement in coronary stent technology. This seventh-generation stent features a unique stent design which utilizes a distinctive CoCr framework composed of struts with varying widths and integrates radiopaque markers into its structure.
Furthermore, the stent features a specialized radiopaque enhanced metal spring tip designed for navigating through complex anatomies. These design elements are intended to equip physicians with precise positional information, facilitating accurate stent placement during PCI procedures.
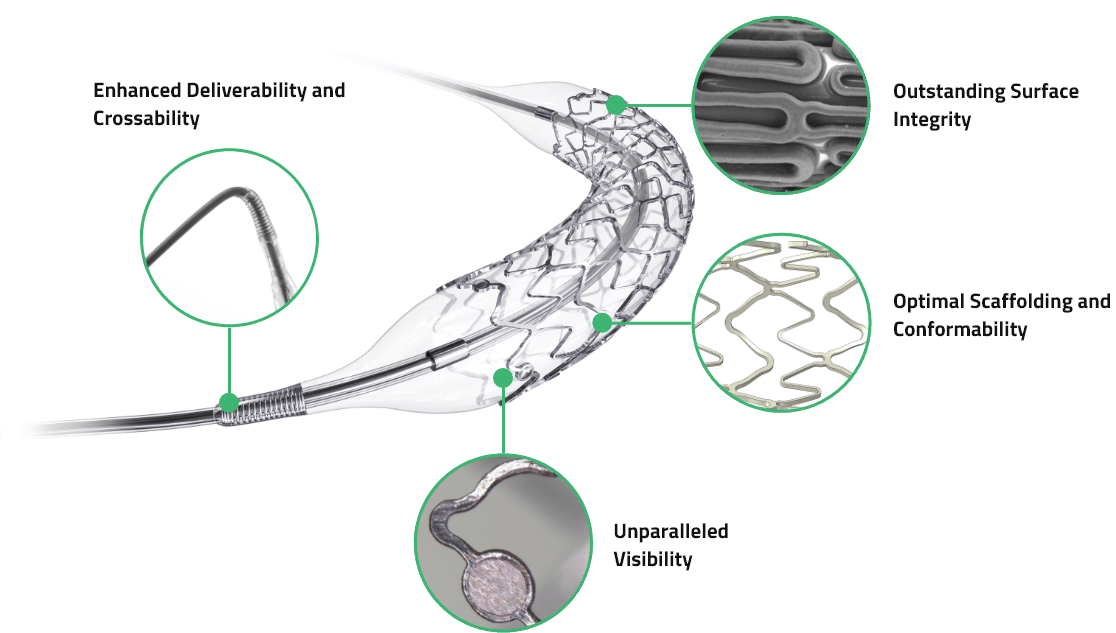
Key Features
Enhanced Deliverability and Crossability
Superior deliverability with a unique and flexible metal spring tip
Unparalleled Visibility
Reinforced radiopaque tip and stent markers for accurate delivery and stent positioning
Outstanding Surface Integrity
Elastomeric coating and outstanding surface integrity providing a uniform rapamycin analogue drug elution
Optimal Scaffolding and Conformability
Unique stent design providing optimal flexibility and radial strength resulting in superior scaffolding and vessel conformability
Clinical Overview
5.4%
n=958
Target Lesion Failure
1-yr BIONICS trial results
0.4%
n=958
Stent Thrombosis (Definite+Probable)
1-yr BIONICS trial results
3.2%
n=958
Target Vessel Myocardial Infraction
1-yr BIONICS trial results
Testimonials
Kaplan Medical Center Heart Institute, Rehovot, Israel
Prof. Michael Jonas
Director of Cardiac Catheterization and Structural Heart Disease Services
Wolfson Medical Center, Holon, Israel
Prof. Haim Danenberg
Head of Interventional Cardiology
Major Regulatory Approvals
- FDA
- CE Mark
Technical Specifications
Our proprietary stent design is paradigm shift in cardiovascular stening
WiZeCell™
Stent Design
Adaptive cell size designed for optimal scaffolding and uniform drug dosing while preventing tissue prolapse.
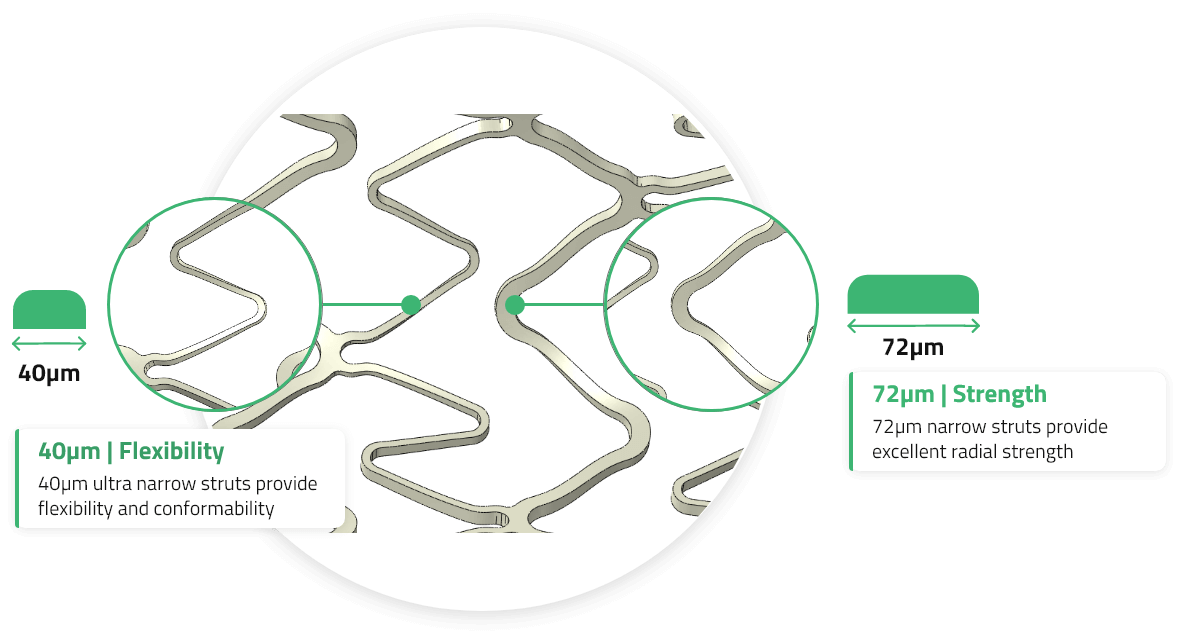
Lower is Better
Low Metal Footprint
Low cobalt-chromium footprint with ultra-narrow 40µm and 72µm struts.
Tissue Prolapse
Scaffolding & Conformability
EluNIR PERL’s optimized cell size ensures uniform scaffolding and prevents tissue prolapse and strut overlap.
Design Features
Low metal footprint cobalt-chromium design
Optimized side branch access
Exceptional Scaffolding and Conformability
Metal Spring Tip
Medinol’s patented Flexx² technology is based on a tapered spring tip designed to eliminate tip flare out and buckling while enabling simultaneous flexibility and pushability. Now with enhanced radiopacity to facilitate positioning and navigability.

Enhanced Metal Spring Tip
Superior Deliverability
Eliminates flare out and buckling
Enhanced Pushability & Flexibility
Enhanced Tip Radiopacity
Tip Visibility
Purposefully designed spring tip for superior deliverability in challenging anatomies
Elastomeric Coating
Long Term Durability
The EluNIR’s novel coating has elastic properties that resists cracking and is designed to maintain surface integrity providing controlled drug elution.
Optimal combination of coating and process design offers predictable and uniform release of Ridaforolimus.
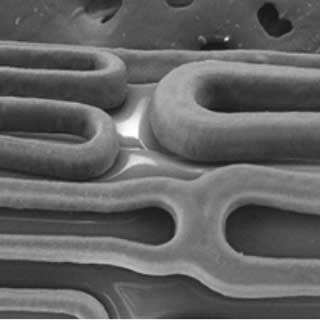
Crimped
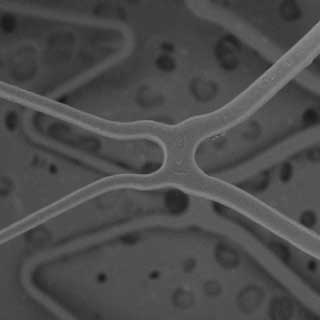
At Nominal Pressure
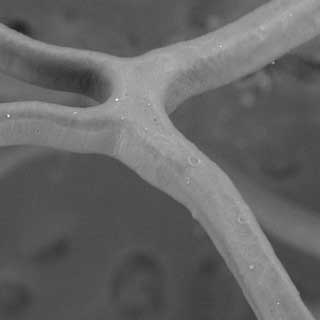
At Rated Burst Pressure
Clinical Data
Medinol Drug-Eluting Stents
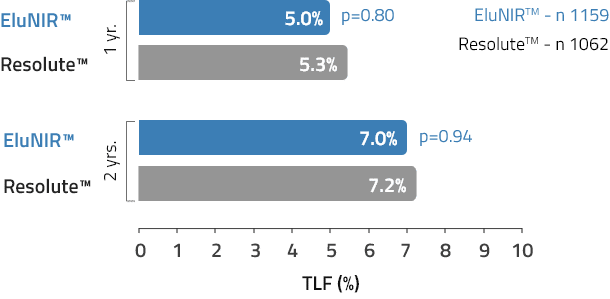
TLF
Target Lesion Failure
The efficacy profile of EluNIR remains strong for a duration of 1 to 2 years when compared to other top-performing drug-eluting stents. EluNIR presents outstanding clinical results with a low TLF of 7.0% at 2 years.
*January 2020 | Randomized Comparison of Ridaforolimus-Eluting and Zotarolimus-Eluting Coronary Stents: 2-Year Clinical Outcomes From the BIONICS and NIREUS Trials.
*January 2020 | Randomized Comparison of Ridaforolimus-Eluting and Zotarolimus-Eluting Coronary Stents: 2-Year Clinical Outcomes From the BIONICS and NIREUS Trials.
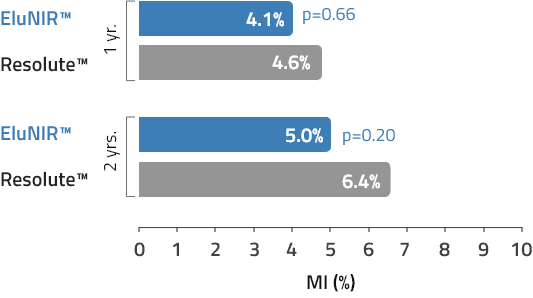
MI
Myocardial Infarction
EluNIR shows similar rates of MI compared to other top drug-eluting stents, with an incidence of 5.0% within a two-year period.
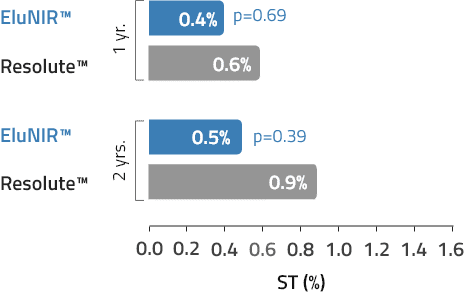
ST
Stent Thrombosis Definite/
Probable
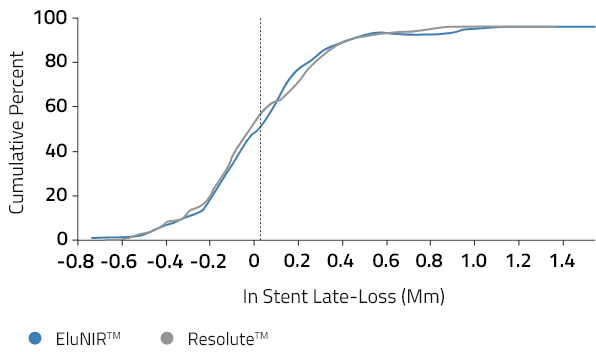
The EluNIR was found non-inferior to Resolute Integrity in a broad, less selected ‘more comers’ population with 0.04mm in-stent late-loss at 6 months with 3.4% target lesion failure at 12 months.
*January 2020 | Randomized Comparison of Ridaforolimus-Eluting and Zotarolimus-Eluting Coronary Stents: 2-Year Clinical Outcomes From the BIONICS and NIREUS Trials
*January 2020 | Randomized Comparison of Ridaforolimus-Eluting and Zotarolimus-Eluting Coronary Stents: 2-Year Clinical Outcomes From the BIONICS and NIREUS Trials.
In Stent Late Loss
Cumulative Distribution Function
EluNIR was found non-inferior to Resolute Integrity in a broad, less selected ‘more comers’ population with 0.04mm in-stent late-loss at 6 months.
Case Studies
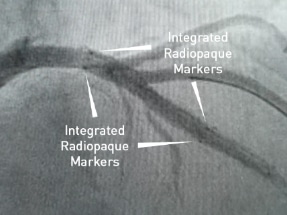
EluNIR PERL First-in-Patient Implantation
Prof. Haim Danenberg, MD
First implantation of the EluNIR-PERL elastomeric Drug Eluting Stent, the only DES with two radiopaque markers at each end of the stent.
Full Article
Ordering Information
| Ø(mm) | Lengh (mm) | 8 | 12 | 15 | 17 | 20 | 24 | 28 | 33 | 38 | 44 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.25 | ELM225R08IN | ELM225R12IN | ELM225R15IN | ELM225R17IN | ELM225R20IN | ELM225R24IN | ELM225R28IN | ELM225R33IN | |||
| 2.5 | ELM250R08IN | ELM250R12IN | ELM250R15IN | ELM250R17IN | ELM250R20IN | ELM250R24IN | ELM250R28IN | ELM250R33IN | |||
| 2.75 | ELM275R08IN | ELM275R12IN | ELM275R15IN | ELM275R17IN | ELM275R20IN | ELM275R24IN | ELM275R28IN | ELM275R33IN | ELM275R38IN | ELM275R44IN | |
| 3 | ELM300R08IN | ELM300R12IN | ELM300R15IN | ELM300R17IN | ELM300R20IN | ELM300R24IN | ELM300R28IN | ELM300R33IN | ELM300R38IN | ELM300R44IN | |
| 3.5 | ELM350R08IN | ELM350R12IN | ELM350R15IN | ELM350R17IN | ELM350R20IN | ELM350R24IN | ELM350R28IN | ELM350R33IN | ELM350R38IN | ELM350R44IN | |
| 4 | ELM400R08IN | ELM400R12IN | ELM400R15IN | ELM400R17IN | ELM400R20IN | ELM400R24IN | ELM400R28IN | ELM400R33IN | ELM400R38IN | ELM400R44IN |



