
Sirolimus Eluting Coronary Stent System
World's Longest Studied Drug Eluting Stent
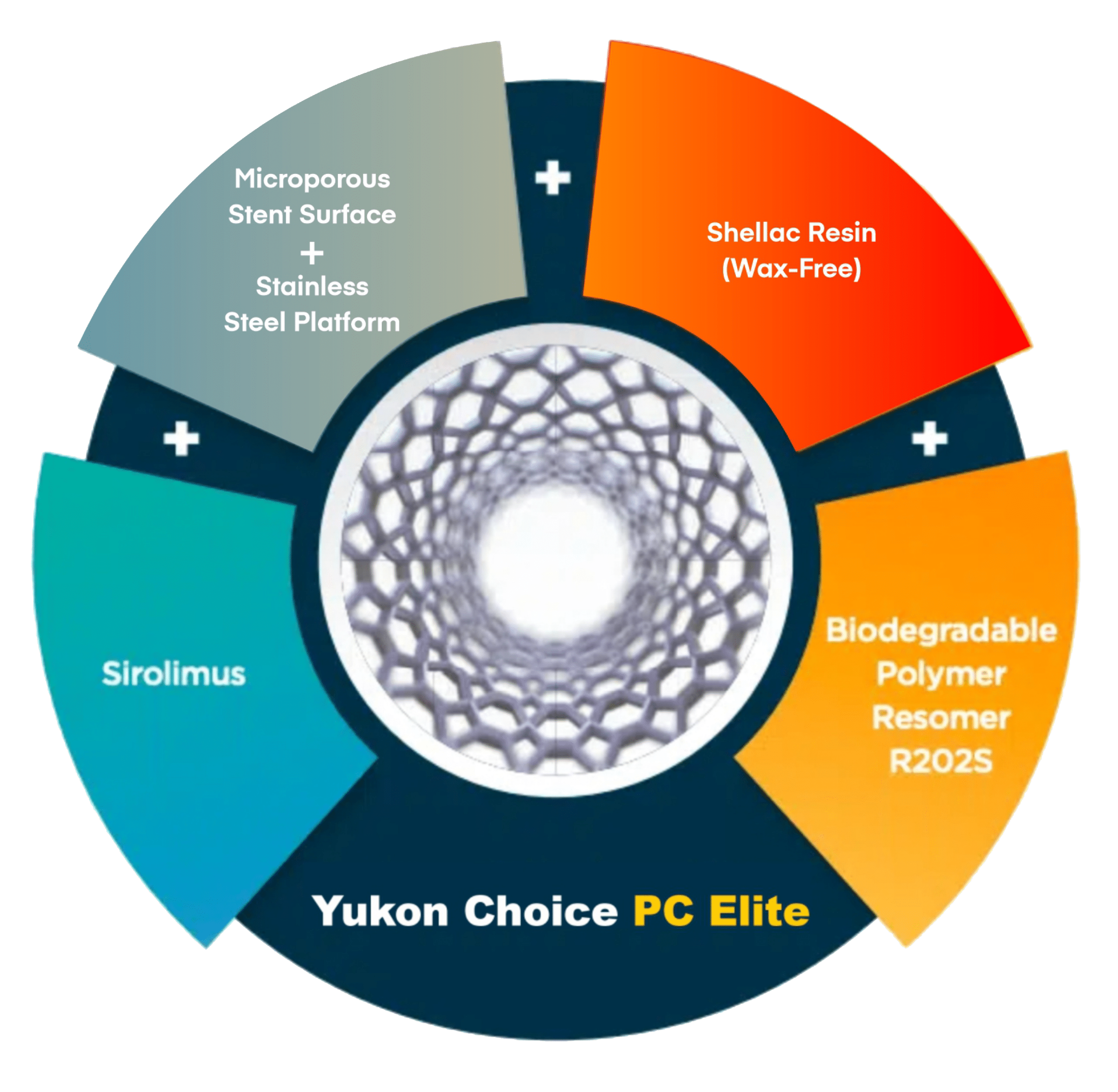
New generation DES providing synergy of Biodegradable Polymer with microporous surface to enhance optimal performance.

Less Polymeric Load Compared to Other DES
- One million pores per cm2 with average depth of 2μm ensures optimum drug release with 1/4th of polymeric load as compared to other DES.
- Shellac Resin (Wax-free) ensures better polymer-drug binding with negligible polymer flaking during stent expansion
Clinical Studies
![]() Yukon Choice PC is recommended by ESC guidelines 2018 on the basis of large randomized trials where primary end points were achieved.
Yukon Choice PC is recommended by ESC guidelines 2018 on the basis of large randomized trials where primary end points were achieved.
![]() Yukon Choice PC showed equivalent efficacy & better safety in terms of stent thrombosis compared to Xience & Cypher at 5 years follow up.
Yukon Choice PC showed equivalent efficacy & better safety in terms of stent thrombosis compared to Xience & Cypher at 5 years follow up.
![]() At four years follow up, Yukon Choice PC shows reduction of risk by 50% in Definite Stent Thrombosis & by 78% in Very Late stent thrombosis as compared to the first generation DES with similar efficacy.
At four years follow up, Yukon Choice PC shows reduction of risk by 50% in Definite Stent Thrombosis & by 78% in Very Late stent thrombosis as compared to the first generation DES with similar efficacy.
![]() In Pre-clinical trial, it was seen that Yukon Choice PC with microporous surface reduced the amount of polymeric load to 1/4th and is assured with consistent kinetics & low inflammation.
In Pre-clinical trial, it was seen that Yukon Choice PC with microporous surface reduced the amount of polymeric load to 1/4th and is assured with consistent kinetics & low inflammation.
![]()
At 10 years, Yukon Choice PC showed equivalence in terms of MACE rate, Mortality and TLR rate compared to Xience and a superiority over Cypher for the same outcomes
Yukon Choice PC showed the lowest rate of definite or probable stent thrombosis with a significant risk reduction than the Cypher stent (50% reduction) and a numerically lower rate as than the ‘Xience’ stent (29% reduction).
![]() At four years follow up, Yukon Choice PC demonstrated reduced rates in stent thrombosis in patients with diabetes mellitus as compared to durable polymer.
At four years follow up, Yukon Choice PC demonstrated reduced rates in stent thrombosis in patients with diabetes mellitus as compared to durable polymer.
![]() At 3 years, Yukon Choice PC proved equivalance to Xience in terms of Late loss, TLR and Primary Composite MACE.
At 3 years, Yukon Choice PC proved equivalance to Xience in terms of Late loss, TLR and Primary Composite MACE.
![]() At 2 years, microporous surface was found to be equally safe as compared to electropolished surface. Rough surfaces showed lesser restenosis rates.
At 2 years, microporous surface was found to be equally safe as compared to electropolished surface. Rough surfaces showed lesser restenosis rates.

