

World's Longest Studied Drug Eluting Stent
The Yukon Choice PC is a drug-eluting stent featuring Sirolimus and biodegradable polylactide (PLA) coating on a stainless-steel platform. Its unique abluminal coating delivers targeted drug release, enhancing endothelialization and minimizing stent thrombosis risks.
Supported by 10 years of robust clinical data, Yukon Choice PC demonstrates proven efficacy and long-term safety outcomes.

Sirolimus
A clinically proven immunosuppressant, Sirolimus inhibits T and B cell activation via mTOR inhibition, reducing IL-2 sensitivity. Its anti-proliferative properties prevent restenosis when used with coronary stents.
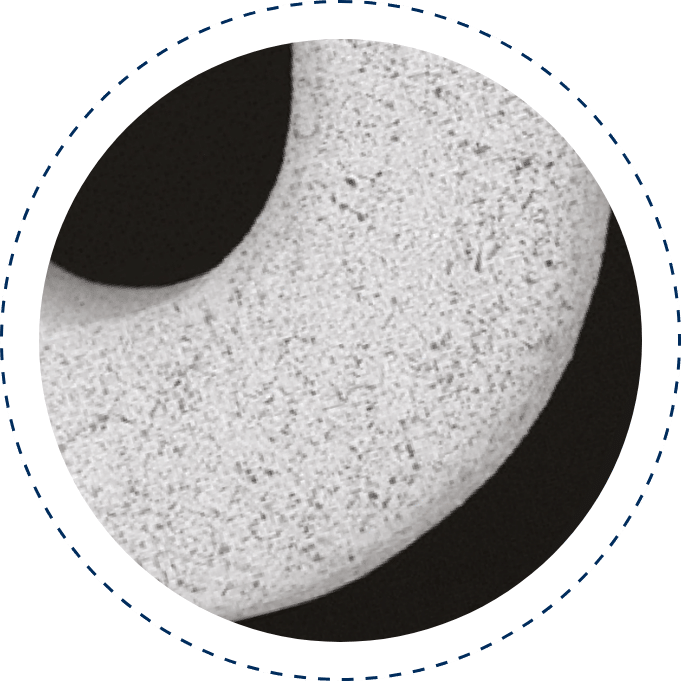
Microporous Surface (PEARL)
The PEARL surface, combined with a biodegradable polymer, enhances endothelialization, reduces restenosis and thrombosis, and ensures controlled drug release, delivering optimal performance as a safe and effective DES technology.
Advanced Stent Design and Delivery System
Homogeneous expansion and increased radial force ensure stable deployment and excellent side branch access.
The soft, flexible tip and high-performance shaft provide superior crossability, maneuverability, and kink resistance, ensuring smooth navigation even in challenging lesions.

Abluminal Coating
Facilitates unidirectional drug release and less systemic exposure, ensuring improved healing & faster endothelialization.
Clinical Studies
10 Years of Robust Data Setting New Standards Longest-Studied DES Technology
![]() In ACS patients, the Yukon stent demonstrated a lower 10-year POCE (65.3% vs. 69% for PP-DES) and a 23% reduced risk of total stent thrombosis.
In ACS patients, the Yukon stent demonstrated a lower 10-year POCE (65.3% vs. 69% for PP-DES) and a 23% reduced risk of total stent thrombosis.
![]() The ISAR-TEST 4 trial’s 10-year follow-up reported the lowest rate of definite/probable stent thrombosis, showing a 50% risk reduction compared to the Cypher stent and a 29% lower rate compared to Xience.3
The ISAR-TEST 4 trial’s 10-year follow-up reported the lowest rate of definite/probable stent thrombosis, showing a 50% risk reduction compared to the Cypher stent and a 29% lower rate compared to Xience.3
![]()
At 10 years, Yukon stent outcomes were comparable to Xience in patients with coronary artery disease, including those with Diabetes Mellitus4, with no significant difference in clinical event rates.
3. Circulation, 138, 00-00. DOI: 10.1161/CIRCULATIONAHA.118.038065
4. J Am Heart Assoc. 2021;10e020165 DOI:10.1161/JAHA.120.020165

