Protégé Paclitaxel Coated Coronary Balloon Dilatation Catheter
Taking DCB technology to a New High
Drug-coated balloon (DCB) treatment is based on a leave-nothing-behind concept. DCB offers homogeneous drug delivery to the vessel wall, immediate drug release without the use of a polymer, the potential of reducing the intensity and duration of antiplatelet therapy, and the absence of residual foreign material in the vessel.

Manufactured by Blue Medical Devices B.V. in the Netherlands EU-MDR Certified
Protégé is powered with technology offering patient benefits with maximum drug available at lesion site.
Introducing our innovative drug-coated balloon (DCB), Protégé with technology that ensures minimal drug loss during transition with patented technology.
Protégé is worlds 1st and only DCB Available in both semi-complaint and non-compliant version.
Protégé is worlds 1st and only DCB Available in both semi-complaint and non-compliant version.
- Linear Expansion with no over growth at high pressure
- NC balloons minimize dissection in complex lesion subset compared to SC balloons*
- For the treatment of ISR and lesions difficult to dilate
- Higher strength than Semi-Compliant DCB**

*Desmet, W. J., De Scheerder, I. K., Barrios, L., & Piessens, J. H. (1997). Catheter Cardiovasc Diagn, 41(1), 5–11.
**Amstutz, C., Behr, J., Krebs, S., Haeberlin, A., Vogel, R., Zurbuchen, A., & Burge, J. (2023). BioMedical Engineering OnLine, 22(94)
Technology Highlights
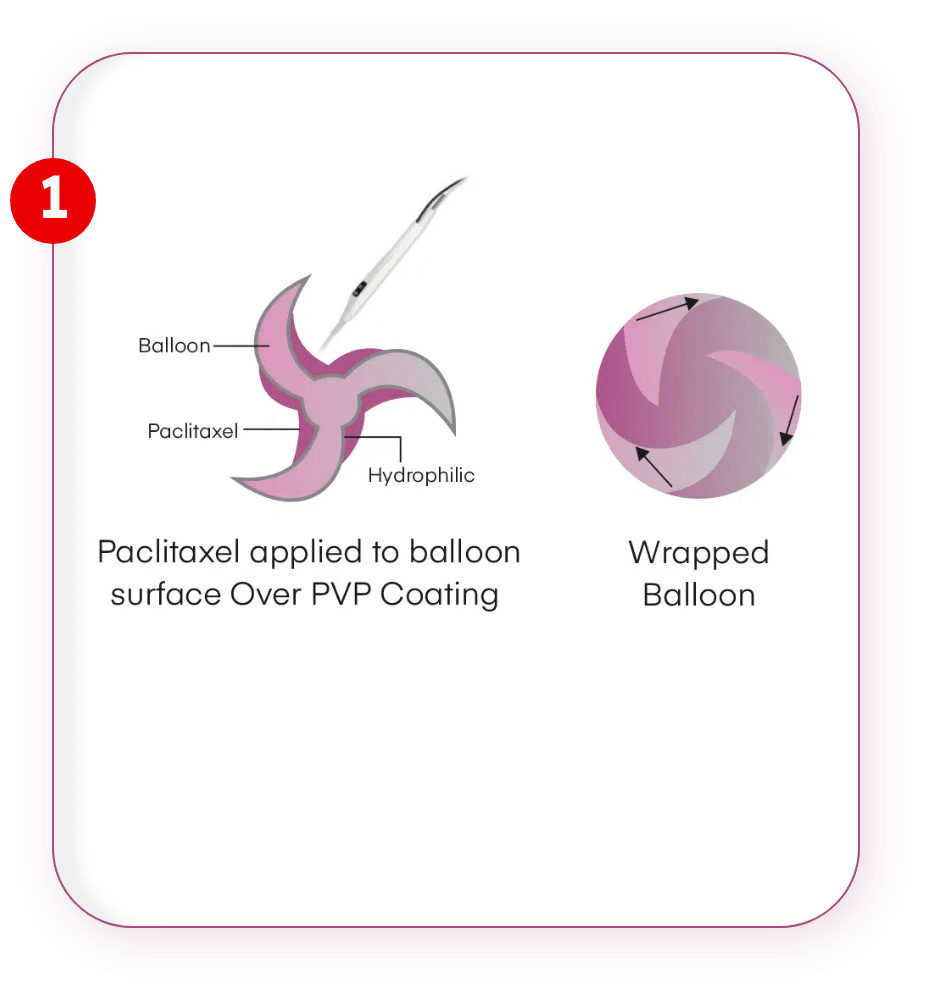
Unique Drug Application
Drug is applied through pipette method within the balloon folds ensuring minimum drug loss during transition.
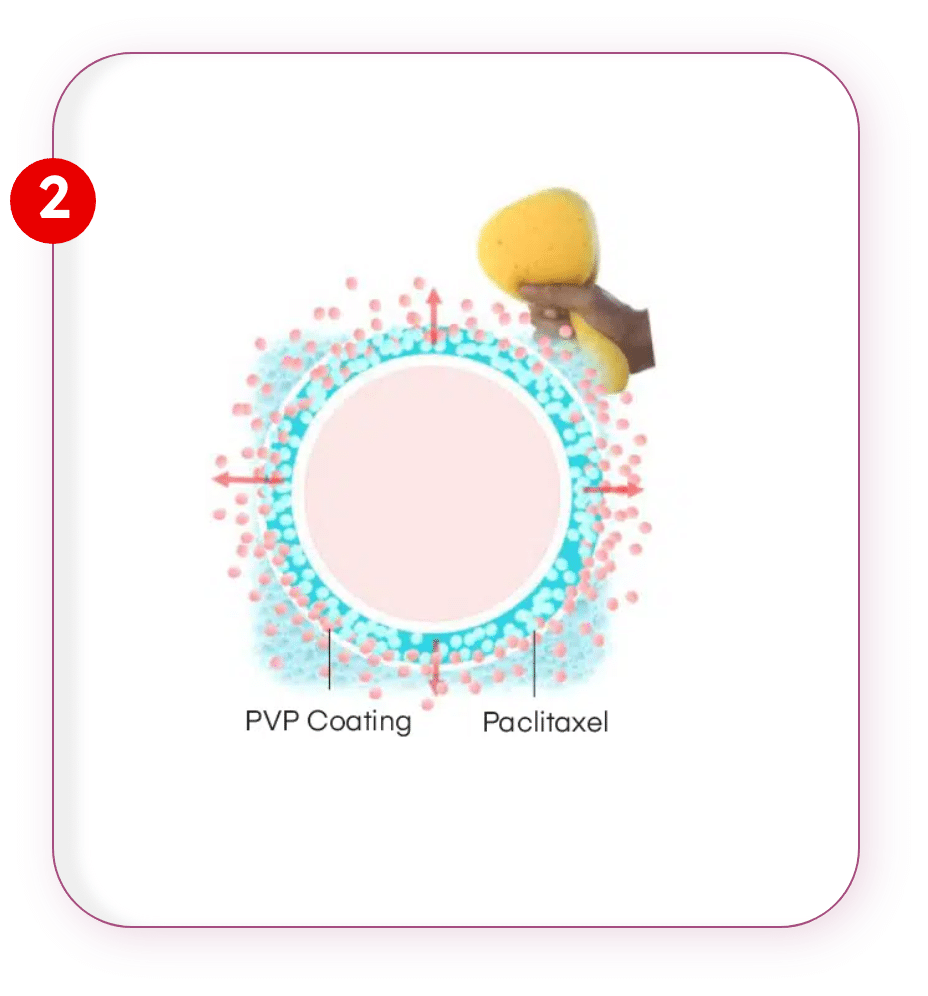
PVP Coating
Paclitaxel is applied over PVP coating which acts as sponge which elutes the Paclitaxel only when pressure is applied.
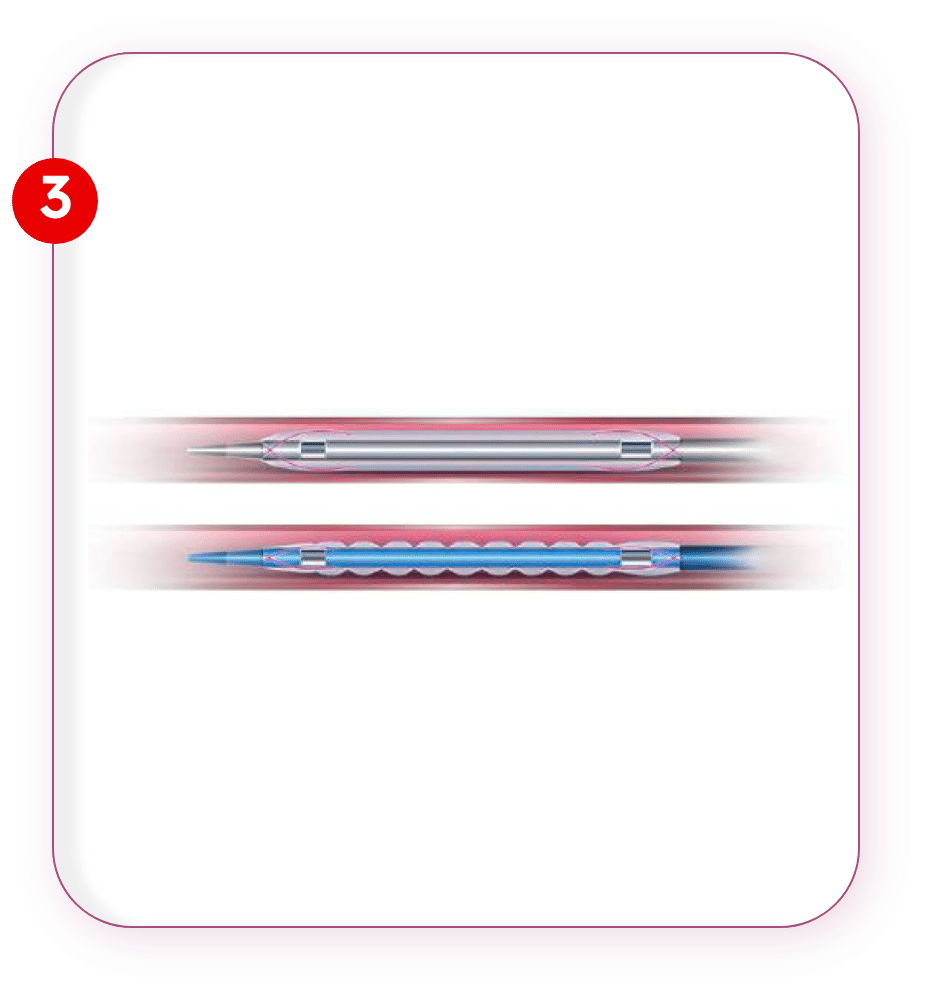
Wing Seal Technology
Wrapped balloon is further crimped & then subjected to a process that creates corrugation on ballon surface offering:
- Minimal drug loss & prevents balloon unfolding during transition.
- Low corrugated balloon profile.
- Low frictional abrasion during balloon advancement.
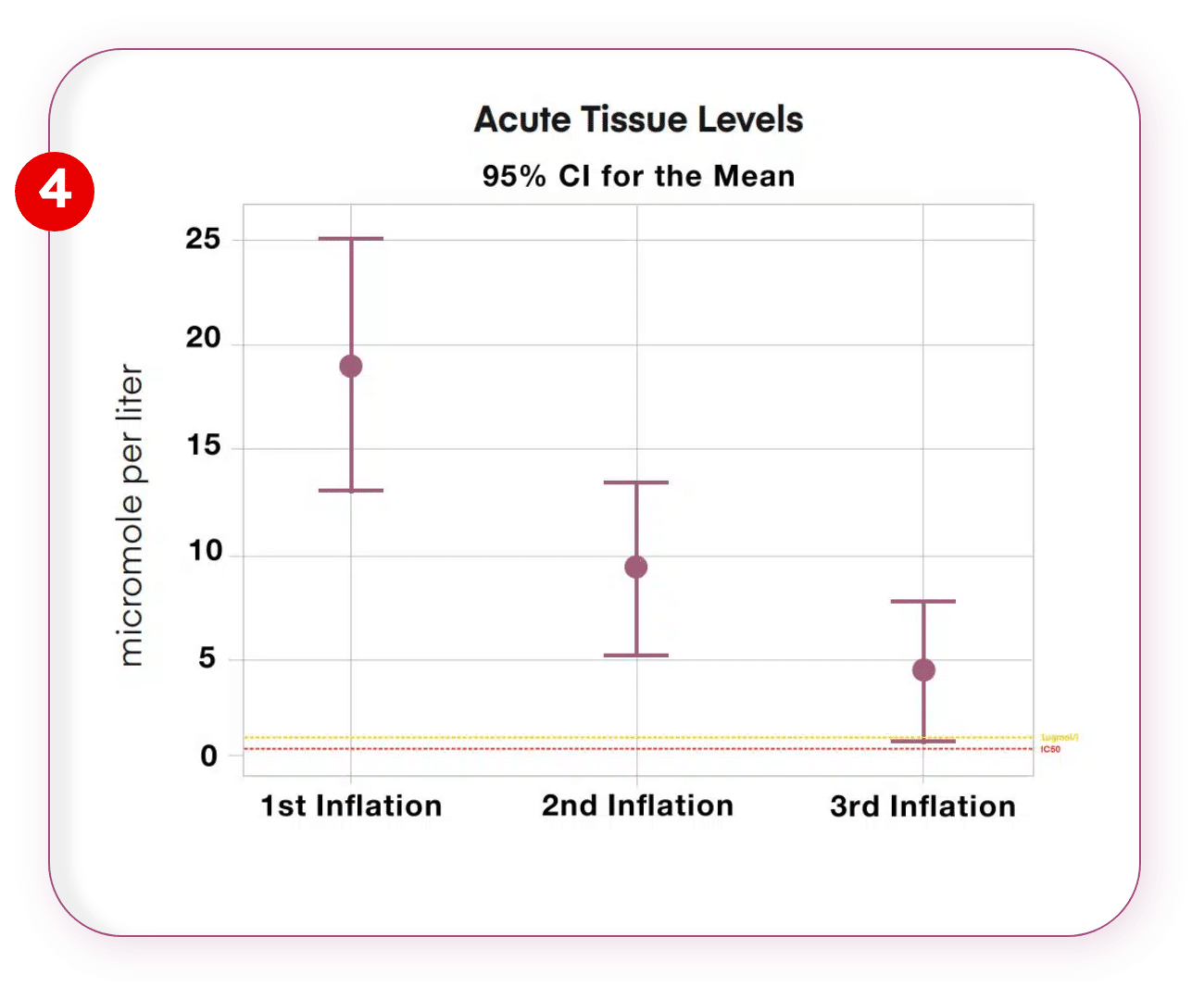
Multiple Inflation
Multiple Drug Release upto 3 times with the same device.
Multiple inflations were performed at a different location, the difference in tissue bound paclitaxel was found to be greater. This was attributed to diffusion characteristics. 3rd Inflation provides 1µmol/l of tissue bound paclitaxel which is minimal optimal dosage to as efficiently as possible inhibit the SMC’s
Clinical Evidence
Highlights of Pearl Registry
Proven safety and efficacy of the Protégé*
In real-world PCI of In-Stent Restenosis (ISR) and De Novo Lesions

- Highly Complex Lesion classified type C - 36%
- Prior PCI - 86.4%
- ISR DES - 60.4%
- Diabetes - 28.3%
*Cheng et al., 2022, J. Invasive Cardiol. 34(6) – Pearl Registry: Paclitaxel-coated balloon in PCI practice.
Study Design
Multicentre, Real World, Single Arm , Observational study conducted at Netherlands
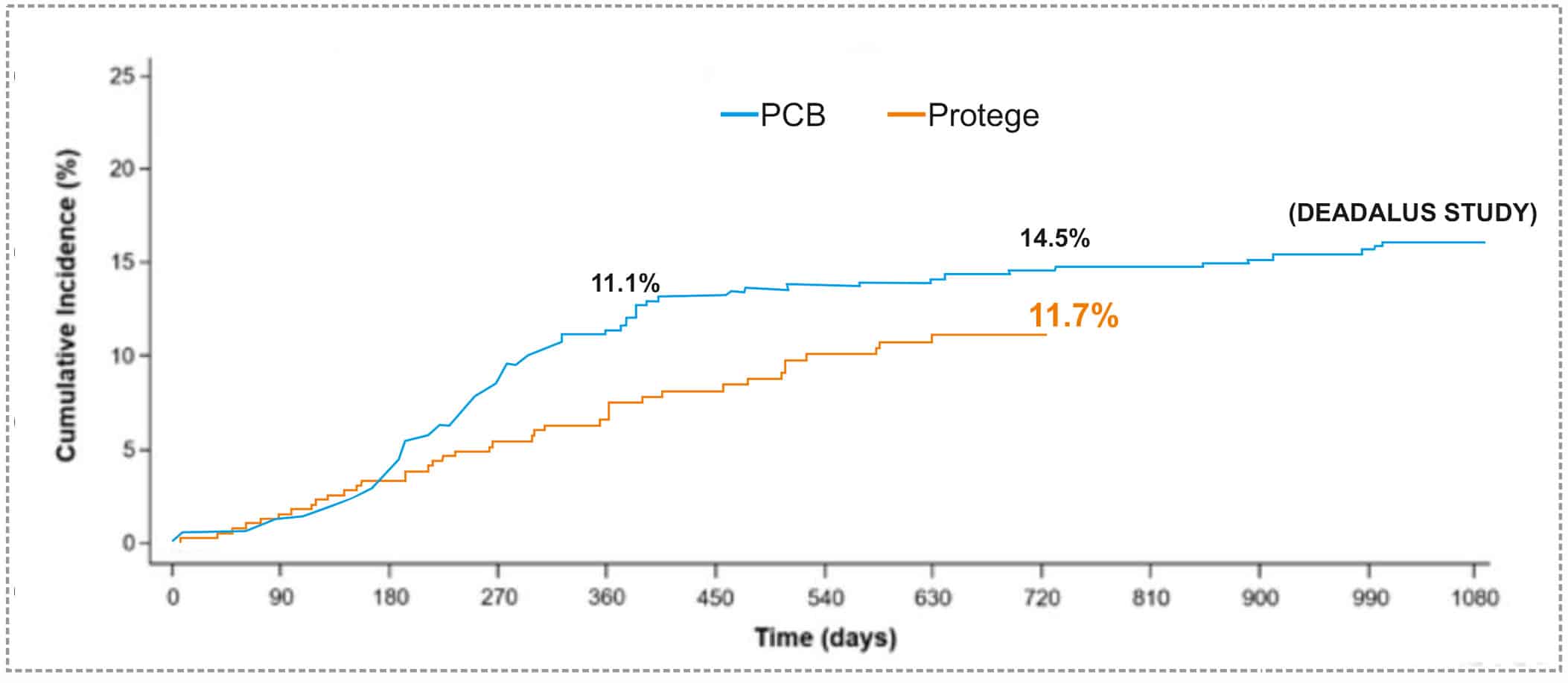
At 2 years MACE driven by TLR in patients treated for ISR was 11.7% & for De Novo Lesions 2.9% which is lower compared to the reported incidence rates in ISR patients (>15%)