The Translumina Yukon drug-eluting stent product family, coated with Rapamycin (Sirolimus) and the biodegradable component polylactide (PLA), has an excellent history of pre-clinical and long-term clinical results.[1-10]
The Yukon Chrome PC has the identical coating technique and coating properties (dosage, thickness) like the clinically proven Yukon Choice PC. In two independent trials ISAR-TEST 3 and ISAR-TEST 4 the Yukon DES platform showed improved longterm performance compared to 1st. Generation DES over a time period of 10 years clinical follow-up in the randomized controlled clinical trial.[3,4]
Clinical data, published by G. Stefanini et al [5], show the excellent long-term outcome of the Yukon Choice PC in a meta-analysis, comparing the clinical outcome after 4 years in more than 4000 patients with the Cypher stent. This analysis shows for the first time that the definite Very Late Stent Thrombosis (VLST) can be reduced statistically significant by using the biodegradable PLA polymer coating technology of the Yukon DES. An additional sub-group analysis shows also benefit in difficult patient groups like diabetics and patients with acute myocardial infarct.[6-7]
Due to this excellent clinical outcome the Yukon DES technology is recommended by the latest ESC guidelines for myocardial revascularization.[8] Yukon Chrome PC now transfers this unique technology to the latest Translumina CoCr-stent platform featuring thin struts and a highly flexible 2-Connector stent design.
Resources
Product Brochure
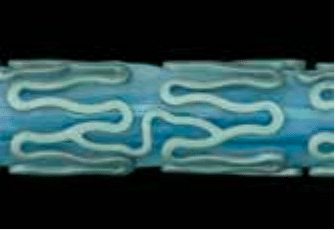
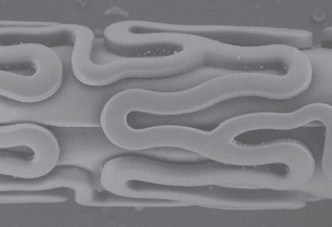
Figure 1: Optical and Electron Microscope Pictures of the Yukon Choice PC. The unique microporous stent surface is coated abluminal with Sirolimus and PLA. The PLA ensures a better binding of the Sirolimus to the microporous stent surface and controls the release of the drug. The PLA is fully degradable according to the Krebs cycle.
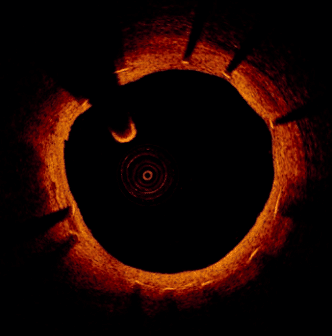
Figure 2: OCT follow-up 3 years after implantation of a Yukon Choice PC.
Published Pre-clinical and BMS Data[1-2]
Extensive pre-clinical evaluations prove the safety of Yukon Choice PC over BMS and conventional DES:
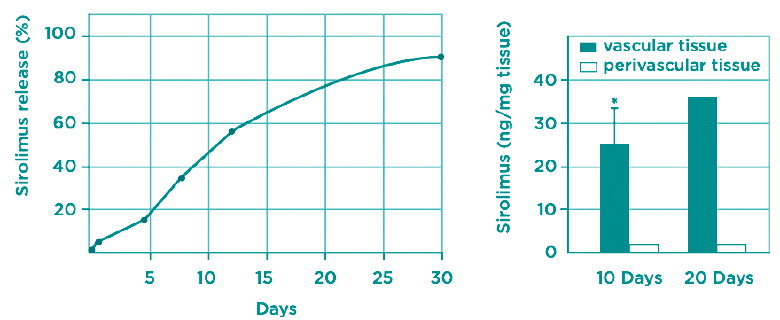
Yukon DES coating shows a release of sirolimus up to 4 weeks with a significant tissue concentration in the arterial segments.
The microporous surface shows a trend towards a reduced rate of binary restenosis with equivalent safety, which proves that it is safe and feasible to use as a drug reservoir for a DES.

Comparison of smoot (electropolished) stent surface (A) and rough (microstructured) stent surface (B). Magnification, 500x
Meta-Analysis Data and ESC Recommendation[5-8]
One of the largest meta-analysis involving more than 4000 patients, which compared biodegradable polymer based DES with permanent polymer based DES demonstrated the long term excellent safety profile of the Yukon Choice PC up to 4 years.
At 4 years follow-up, the Yukon Choice PC shows a reductionof risk by 50% in definite Stent Thrombosis and by 78% in Very Late Stent Thrombosis (VLST) compared to First Generation DES without compromising on efficacy. Additionally, the Yukon Choice PC achieved highest recommendations in the latest ESC Guidelines for myocardial revascularization (2018) due to the excellent clinical outcome.
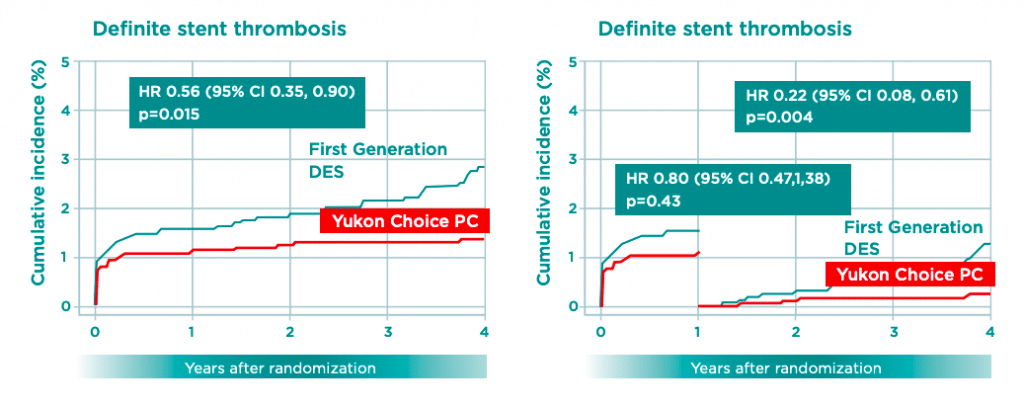
Biodegradable Polymer technology enhances the long-term safety when compared to permanent polymer DES.
Excellent 5 and 10 year long-term clinical data[9-10]
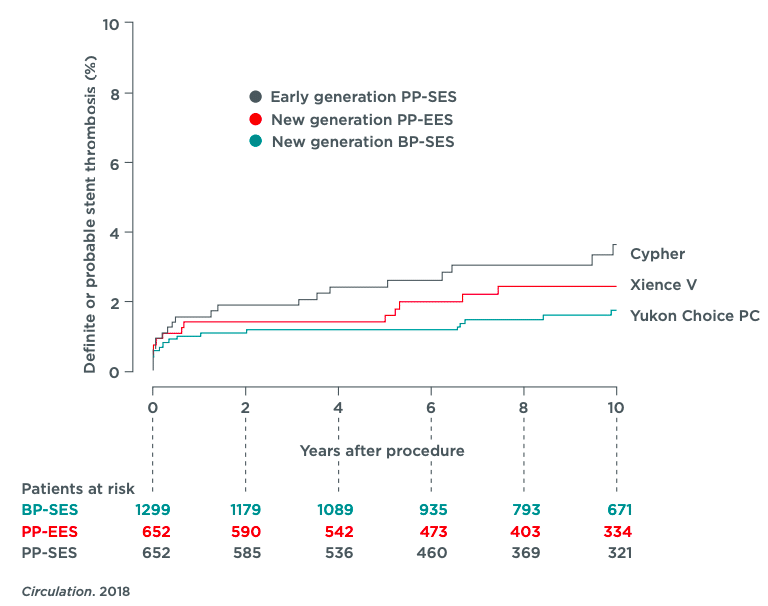
The final 10 year long-term clinical follow-up of the ISAR-TEST 4 randomized controlled clinical trial showed excellent safety and efficacy data for the Yukon Choice PC when compared with the Cypher and Xience V stent. The definite and probable stent thrombosis was only 1,8% for the Yukon compared to 2,5% and 3,7% for the 2 permanent polymer coated competitor DES.

ARC definite or probable stent thrombosis
The new stent delivery system
A CoCr Stent – designed for all lesions
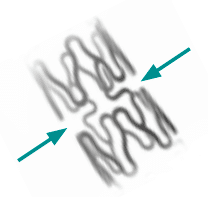
The new design with only 2 connector struts guarantees maximum flexibility and side branch access.
With a cell circumference of up to 18,5 mm the Yukon Chrome PC allows perfect side branch access which is essential for bifurcation stenting.
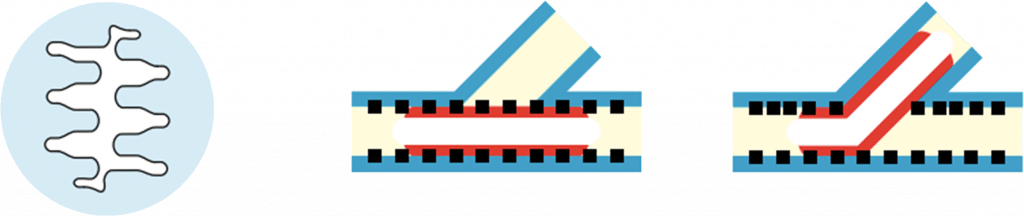


The luer is designed with an integrated kinking protection. The flexible tip ensures perfect crossability and trackability.
Scanning Electron Microscope picture of the unique microporous surface
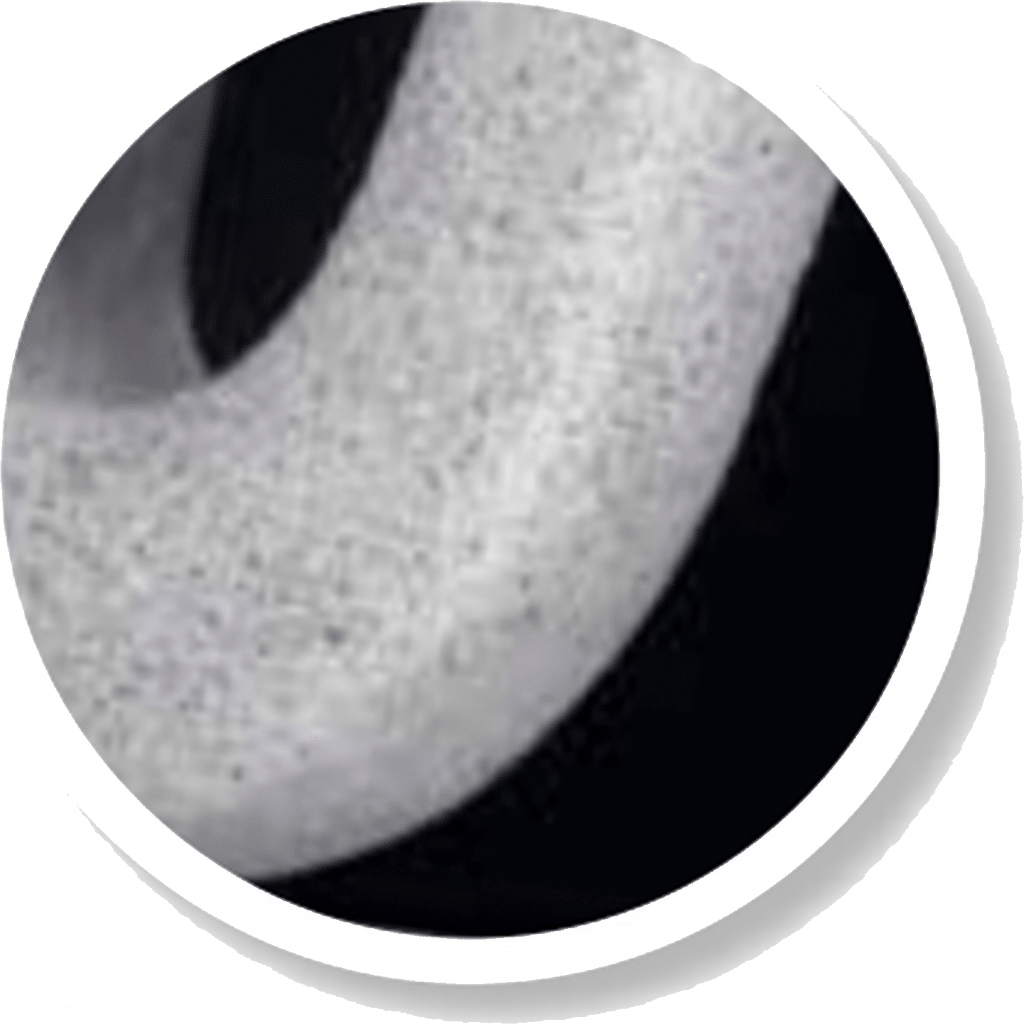
• average micro pore depth of approx. 2 μm
• 100% pore coverage of the surface
• increased radial force
• good side branch access
• flexible and deliverable
• strut thickness only 68 µm for the Small Vessel Designs
Crossing profile (Ø 2,5 mm) | 0,91 … 0,93 mm (Ø 2,0 mm … 2,5 mm; SV) |
0,97 … 1,13 mm (Ø 2,75 mm … 4,0 mm; MV) | |
Strut thickness | 68 μm (SV), |
79 µm (MV) | |
Metallic surface area | 9,1 – 14,9% |
Balloon marker material | Platinum / Iridium |
Entry profile | 0,016″ / 0,41 mm |
Proximal shaft diameter | 1,9 F |
Distal shaft diameter | 2,7 F |
Recommended guide wire | 0,014″ |
Guiding Catheter | min. 5 F |
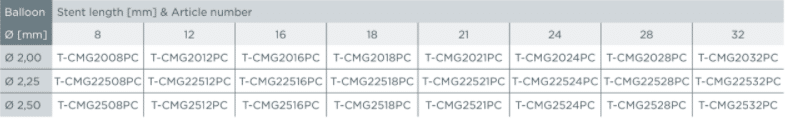
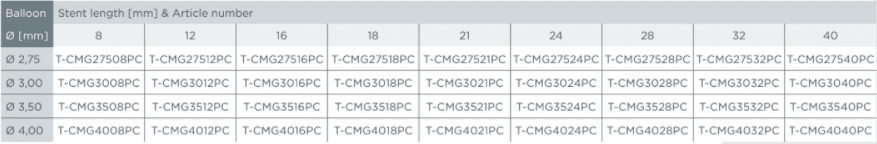
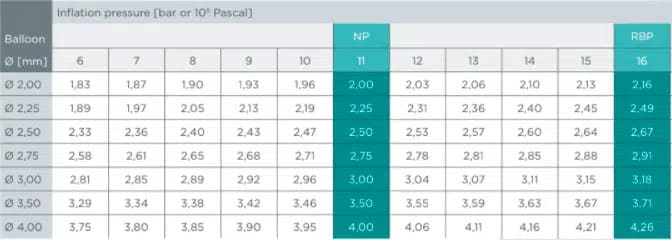
Product matrix / Ordering information
Small vessel design (SV)
Medium vessel design (MV)
Compliance chart
2. Microporous Stent BMS study: Dibra et al, Cath. Cardiovasc. Interv., 2005; 65, 374-380.
3. ISAR-TEST 4 trial, 1 year data (comparison with Cypher / Xience V): R.Byrne et al, European Heart Journal, 2009 ; 30, 2441-2449.
4. ISAR-TEST 4 trial, 3 year data (comparison with Cypher / Xience V): R.Byrne et al, JACC, 2011 ; 58, 1325-1331.
5. Meta-Analysis ISAR-TEST 3 + 4, LEADERS, 4 years follow-up (Comparison of Yukon Choice PC + Biomatrix versus Cypher): G.Stefanini, European Heart Journal, 2012; 33, 1214-1222.
6. Subgroup-Analysis of the Meta-Analysis with regard to Diabetics: A. de Waha et al, International Journal of Cardiology 2013, 168, 5162-6
7. Subgroup-Analysis of the Meta-Analysis with regard to STEMI patients: A. de Waha et al, Euro-Intervention 2014, published online.
8. F.-J. Neumann et al., 2018 ESC/EACTS Guidelines on Myocardial Revascularization, European Heart Journal (2018) 00, 1–96 and supplement.
9. S.Kufner et al, 5-year long-term follow-up of the ISAR-TEST 4 trial (Yukon Choice PC versus Cypher / Xience V), EuroIntervention 2016; 11:1372-1379.
10. S.Kufner et al, 10-year long-term follow-up of the ISAR-TEST 4 trial (Yukon Choice PC versus Cypher / Xience V), Circulation, 2018; 139, 325–333.





